
Researchers from Pacific Northwest National Laboratory (PNNL) achieved a breakthrough by using a common food and medicine additive, β-cyclodextrin, derived from starch, in flow battery design.
The battery maintained ability to store and release energy for more than a year of continuous charge and discharge.
A remarkable experiment revealed how a simple food and medicine additive could boost both the capacity and lifespan of an advanced flow battery design.
β-cyclodextrin additive accelerated electrochemical reaction
The researchers conducted a series of experiments. They fine-tuned the chemical composition of the battery system. And achieved an impressive 60 percent increase in peak power.
They then proceeded to cycle the battery continuously for over a year, only concluding the experiment when the plastic tubing failed.
Remarkably, the flow battery exhibited minimal capacity loss throughout the entire duration of the experiment.
This represents the first laboratory-scale flow battery study to demonstrate continuous usage for over a year with negligible capacity decline.
Notably, the β-cyclodextrin additive also marks the first instance of accelerating the electrochemical reaction involved in storing and releasing energy in the flow battery.
Homogeneous catalysis
This acceleration is achieved through a process called homogeneous catalysis. It refers to a catalytic process where the catalyst and the reactants are present in the same phase, typically as a solution. The process enables efficient and selective transformations in various chemical reactions.
Unlike previous methods that involved applying a solid substance to a surface, the sugar additive operates effectively while dissolved in the solution.
According to Wei Wang, a battery researcher at PNNL and the principal investigator of the study, this innovative approach represents a completely novel method for developing flow battery electrolytes.
By utilizing a distinct catalyst that enhances energy conversion, the study demonstrates the potential of this new technique.
Additionally, the dissolved state of the catalyst within the liquid electrolyte mitigates the risk of solid particles becoming dislodged and causing system contamination. Thus, the approach offers further advantages in terms of system reliability and performance.
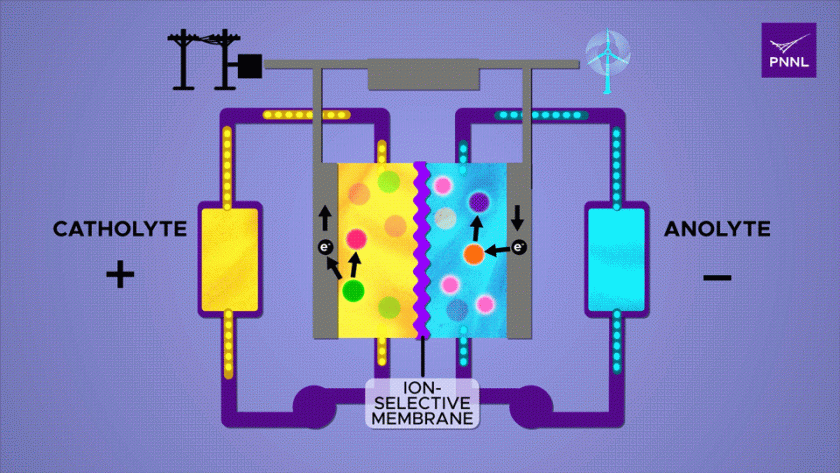
What is a flow battery?
A flow battery, also known as a redox flow battery, is a type of rechargeable battery that stores electrical energy in liquid electrolyte solutions.
It is a variation of conventional electrochemical batteries where energy is stored in chemical compounds.
Flow batteries, comprise two chambers containing distinct liquids. These batteries charge by means of an electrochemical reaction, which stores energy within chemical bonds.
When connected to an external circuit, they release this stored energy, capable of powering electrical devices.
Unlike solid-state batteries, flow batteries employ a unique design where two external supply tanks continuously circulate liquids, serving as the electrolyte—a vital component comparable to the system’s “blood supply.”
The size of the electrolyte supply tank directly influences the energy storage capacity of the flow battery.
Versatility of flow batteries
Flow batteries have the potential to function as backup generators for the electric grid when scaled up to the size of a football field or even larger.
They play a vital role as a fundamental component of decarbonization strategies, providing a means to store energy generated from renewable sources.
One significant advantage of flow batteries is their versatility in terms of scalability. They can be constructed at various scales, ranging from small lab-bench prototypes, as demonstrated in the PNNL study, to extensive installations spanning the size of an entire city block.
What is the need of these new kinds of flow batteries?
Large-scale energy storage, such as flow battery facilities, acts as an insurance policy for our electrical grid.
During instances of severe weather or high electricity demand that may hinder the power supply to homes and businesses, these energy storage systems can play a crucial role in mitigating disruptions and restoring service.
As the generation of electricity progressively transitions to renewable energy sources like wind, solar, and hydroelectric power, the necessity for such flow battery facilities is anticipated to increase.
Since these renewable sources often generate power intermittently, energy storage provides a solution by storing excess energy until it is required to meet consumer demand.
Although various flow battery designs exist and several commercial installations are in place, current commercial facilities predominantly depend on mined minerals like vanadium.
However, these minerals are expensive and challenging to acquire. Consequently, research teams are actively exploring alternative technologies that utilize readily available, easily synthesized, stable, and non-toxic materials.

Takeaway
The integration of β-cyclodextrin as an additive in flow battery design represents a groundbreaking advancement.
Flow batteries, with their versatility and scalability, are key contributors to grid resilience and the storage of renewable energy. Thus, paving the way for a cleaner and more reliable energy future.



